IV Fluid Disruption Information
REcommendations, resources, & updates regarding the current IV fluid disruption From Hurricane Helene.
For questions, please reach out to us at R7DHRE@unmc.edu
-
Baxter Updates (updated 01.28.25)
01.28.25
Baxter announced that IV solutions production has been restarted on all manufacturing lines at its North Carolina site impacted by Hurricane Helene in October. Baxter said some of the manufacturing lines will require additional time to increase production, and the company expects production to return to pre-hurricane levels early in the first quarter of 2025.
AHA Update: Per a communication from Baxter to its customers, allocations for several U.S. IV products will be increased to 100%. For more information about which product lines are increased, please visit theAHA site.
FDA Update: The Food and Drug Administration Dec. 31 released an alert highlighting a Baxter letter that recommended health care providers not use certain lots of Solution Sets with Duo-Vent Spikes due to a potentially high-risk issue. Baxter reported that some affected products were incorrectly assembled with inverted slide clamps.
The affected products include:
Solution Set with Duo-Vent Spike, lot DR24C22079, expiration March 24, 2026, and lot DR24H23086, expiration Aug. 26, 2026.
Clearlink System Solution Set with Duo-Vent Spike, lot DR24C15109, expiration March 16, 2026.
Continu-Flo Solution Set with Duo-Vent Spike, 2 Luer Activated Valves, Male Luer Lock Adapter with Retractable Collar, lot DR24B21017, expiration Feb. 28, 2026.
-
Multiple facilities, with varying levels of concern, have indicated that the temporary closure of a Baxter manufacturing plant in Marion, N.C., because of damage from Hurricane Helene may impact the supply chain. Conservation and contingency strategies are likely to be needed.
This Baxter facility is a critical supplier of intravenous and peritoneal dialysis solutions, producing approximately 60% of the IV solutions used domestically. Baxter is actively working on remediation efforts and exploring alternative production sites to mitigate the impact. Some alternative vendors have put customers on allocation for protective reasons but intend to ramp up production to mitigate these challenges.
ASPR and the FDA are actively working with alternate manufacturers to increase output. Baxter currently has 500 personnel on site for remediation and expects that number to double next week. -
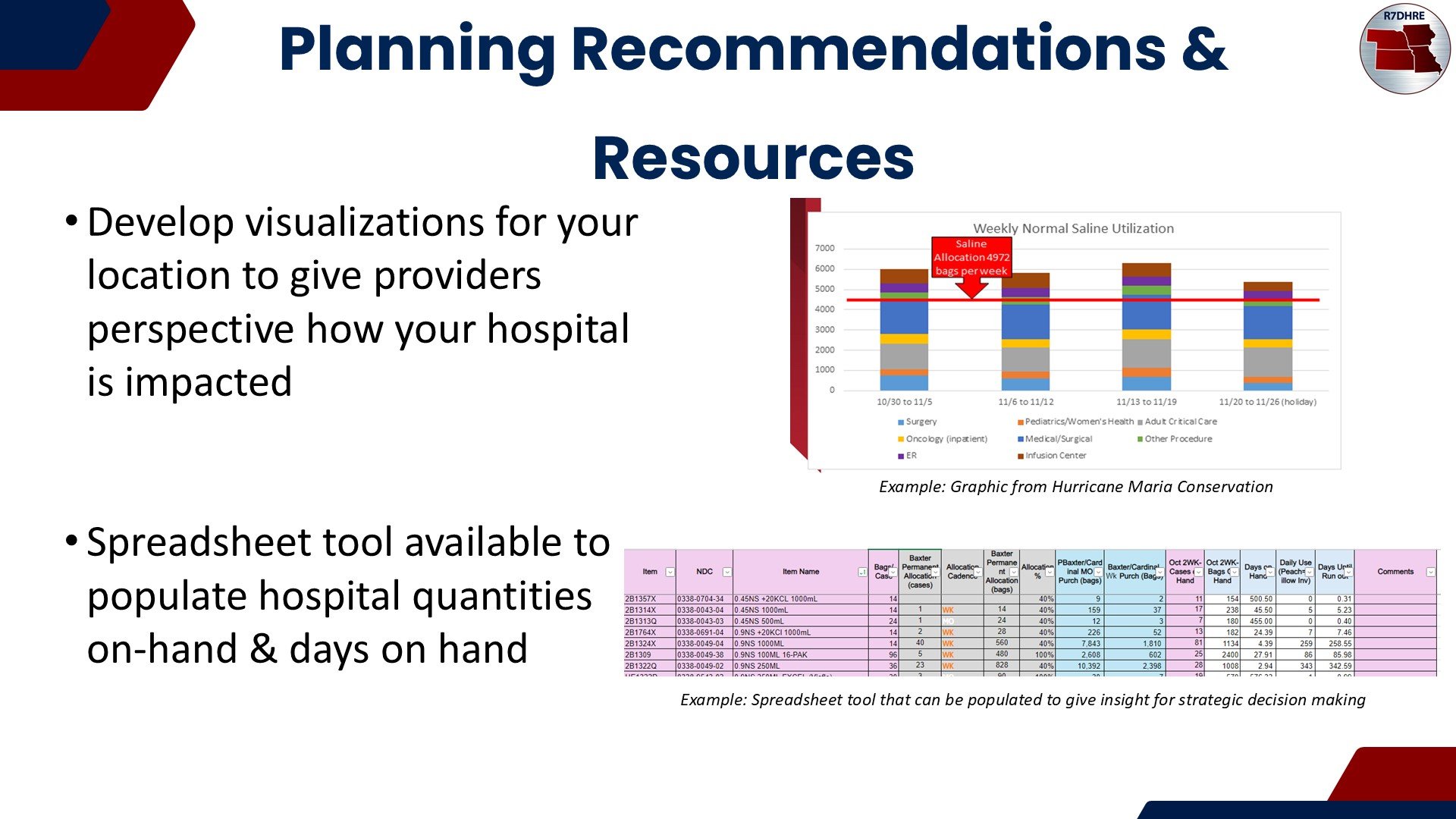
The disruption in supply of these items may impact patient care. A current inventory and use rate of these items can clarify which conservation strategies will prove most helpful. Per Baxter, the current estimate is for this disruption to last 3 months.
Use this spreadsheet, based on your historical data, to determine how many days your current supply will last.
-
Use this spreadsheet, based on your historical data, to determine how many days your current supply will last.
Rehydration Therapy (EMR Build)
ASHP
ASPR
FDA
Vizient
Conservation Support Resources
Intravenous Solution Conservation Strategies, American Society of Health-System Pharmacists (ASHP)
ISMP Safe Practice Guidelines for Adult IV Push Medications 2015
ATS Clinical Recommendations: 10 Steps for Hospitals Facing Intravenous Fluid Shortages 2017
Saline flush and vial shortage, Infusion Nurses Society 2021
Saline Shortages – Many Causes, No Simple Solution NEJM 2018
ASA Suggested Actions on Conservation of IV Solutions During Ongoing Shortage
Interim Strategies of Peritoneal Dialysis Solution Use for Prevalent Patients Undergoing PD
-
FDA Update: To address the current drug shortage, B. Braun is releasing 12 lots of sodium chloride 0.9% (500ml and 1000ml). These lots are associated with primary air volume (headspace) below specification limit which could impact container fluid level monitoring during use.
Baxter updates:
12.19.24
Baxter is making continued strong progress at North Cove and have now restarted 8 of the 10 manufacturing lines at the site, which represent ~85% of the site’s total pre-hurricane capacity.
While the recently restarted lines will require time to ramp up, some of the earlier lines to restart are operating near pre-hurricane levels.
Baxter currently expect to be producing at pre-hurricane levels across the plant early in the first quarter of 2025.
Note: it will take some time for product to flow through the distribution channels. To that end, we currently expect that North Cove’s recovery to full production will translate to removal of allocations for the related product groups during the first quarter of 2025.
Baxter communicated additional allocation increases for several IV product groups to U.S. customers and distributors, effective in phases beginning Dec. 16 and Dec. 30.
U.S. PD Solutions Allocations and Ongoing Patient Support:
Effective Dec. 16, Baxter has increased product allocations for specific peritoneal dialysis (PD) solutions. These allocations have been communicated directly to our provider partners.
Baxter has also significantly expanded new PD patient starts, as communicated with provider partners.
12.05.24:
Baxter is making continued strong progress at our North Cove site, including the initial resumption this week of the following:
All 3L irrigation manufacturing lines
All peritoneal dialysis (PD) solutions manufacturing lines
Note that while production on these lines has restarted, it will take some time for the lines to ramp up and produce at pre-hurricane levels.
Baxter communicated details on allocation increases for several IV product groups to U.S. customers and distributors effective Nov. 26.
11.21.24:
Baxter expects to communicate details on the next planned allocation increases to U.S. customers on Nov. 25th.
Baxter released the first product (1L IV solutions) that was manufactured post-hurricane.
11.14.24:
Second IV solutions manufacturing line restarted
Peritoneal dialysis solutions and irrigation will be next 2 lines to restart in early December
2nd temporary bridge installed at site
10.31.24: Baxter Restarts busiest IV solutions manufacturing line
ASPR Coordinates Airlift Operation to Increase Access and Supply of IV Fluids (10.19.24)
FDA: Hurricane Helene: Baxter’s manufacturing recover in North Carolina(current as of 10.17.24)
On 10.07.24, the American Hospital Association (AHA) sent a letter to President Biden urging certain actions be taken to, “…increase the supply of IV solutions for the nation’s hospitals, health systems, and other health care providers that are already struggling to provide care.”
The letter can be found here: AHA Letter to President Biden
Baxter to Increase Allocations for IV Solution Supplies to Hospitals Effective Oct. 9
"Today on a call hosted by the Department of Health and Humans Services, Heather Knight, Baxter executive vice president and group president of medical products and therapies, announced that Baxter will be changing allocation for some IV solution supplies from 40% to 60% effective tomorrow, Oct. 9. In addition, Baxter expects to be at 70% by the end of October and 90% to 100% by the end of the year. We anticipate Baxter will notify customers later tonight or tomorrow, and we will provide updates as they become available. The AHA appreciates our ongoing partnership and collaboration with Baxter and HHS to help mitigate the impact to patients."
Click on the image below to download our recommendations guide